Polychlorotrifluoroethylene
| |
| Names | |
|---|---|
| Other names
Poly(1-chloro-1,2,2-trifluoroethylene)
Poly(ethylene trifluoride chloride) Polymonochlorotrifluoroethylene Poly(trifluoroethylene chloride) Poly(chlorotrifluoroethylene) Poly(trifluorochloroethene) Poly(chlorotrifluoroethene) Poly(trifluorovinyl chloride) Poly(vinyl trifluorochloride) Kel-F 300; Kel-F 81 | |
| Identifiers | |
| Abbreviations | PCTFE, PTFCE[1] |
| ChemSpider |
|
| ECHA InfoCard | 100.120.473 |
| MeSH | Polychlorotrifluoroethene |
CompTox Dashboard (EPA)
|
|
| Properties | |
| (C2ClF3)n°° | |
| Molar mass | Variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
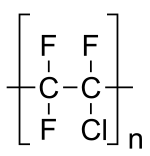
Polychlorotrifluoroethylene (PCTFE or PTFCE) is a thermoplastic chlorofluoropolymer with the molecular formula (CF2CClF)n, where n is the number of monomer units in the polymer molecule. It is similar to polytetrafluoroethene (PTFE), except that it is a homopolymer of the monomer chlorotrifluoroethylene (CTFE) instead of tetrafluoroethene. It has the lowest water vapor transmission rate of any plastic.[2]
History
[edit]It was discovered in 1934[3][4] by Fritz Schloffer and Otto Scherer who worked at IG Farben Company, Germany.[5]
Trade names
[edit]After World War II, PCTFE was commercialized under the trade name of Kel-F 81 by M. W. Kellogg Company in early 1950s.[6] The name "Kel-F" was derived from "Kellogg" and "fluoropolymer", which also represents other fluoropolymers like the copolymer poly(chlorotrifluoroethylene-co-vinylidene fluoride) (Kel-F 800).[7] These were acquired by 3M Company in 1957.[6] 3M discontinued manufacturing of Kel-F by 1996.
PCTFE resin is now manufactured under different trade names such as Neoflon PCTFE from Daikin, Voltalef from Arkema, and Aclon from Honeywell. PCTFE films are sold under the tradename Aclar by Honeywell.[8] Other current and former trade names of PCTFE include Hostaflon C2 from Hoechst, Fluon from ICI, Plaskon from Allied Chemical Corporation, Halon from Ausimont USA,[9][10] and Ftoroplast-3 in the USSR and Russian Federation.[11]
Synthesis
[edit]PCTFE is an addition homopolymer. It is prepared by the free-radical polymerization of chlorotrifluoroethylene (CTFE)[12] and can be carried out by solution, bulk, suspension and emulsion polymerization.[13]
Properties
[edit]PCTFE has high tensile strength and good thermal characteristics. It is nonflammable[14] and the heat resistance is up to 175 °C.[15] It has a low coefficient of thermal expansion. The glass transition temperature (Tg) is around 45 °C.[1]
PCTFE has one of the highest limiting oxygen index (LOI).[16] It has good chemical resistance. It also exhibits properties like zero moisture absorption and non wetting.[15][17]
It does not absorb visible light. When subjected to high-energy radiation, it undergoes degradation like PTFE.[18] It can be used as a transparent film.[14]
The presence of a chlorine atom, having greater atomic radius than that of fluorine, hinders the close packing possible in PTFE. This results in having a relatively lower melting point among fluoropolymers,[19] around 210–215 °C.[2]
PCTFE is resistant to the attack by most chemicals and oxidizing agents, a property exhibited due to the presence of high fluorine content. However, it swells slightly in halocarbon compounds, ethers, esters and aromatic compounds.[2] PCTFE is resistant to oxidation because it does not have any hydrogen atoms.[20]
PCTFE exhibits a permanent dipole moment due to the asymmetry of its repeating unit. This dipole moment is perpendicular to the carbon-chain axis.[21]
Differences from PTFE
[edit]PCTFE is a homopolymer of chlorotrifluoroethylene (CTFE), whereas PTFE is a homopolymer of tetrafluoroethylene. The monomers of the former differs from that of latter structurally by having a chlorine atom replacing one of the fluorine atoms. Hence each repeating unit of PCTFE have a chlorine atom in place of a fluorine atom. This accounts for PCTFE to have less flexibility of chain and hence higher glass transition temperature. PTFE has a higher melting point and is more crystalline than PCTFE, but the latter is stronger and stiffer. Though PCTFE has excellent chemical resistance, it is still less than that of PTFE.[22] PCTFE has lower viscosity, higher tensile strength and creep resistance than PTFE.[1]
PCTFE is injection-moldable and extrudable, whereas PTFE is not.[1]
Applications
[edit]PCTFE is used primarily for two properties: water repulsion and chemical stability.
PCTFE films are used as protective layers against moisture. These include:
- moisture barriers in pharmaceutical blister packaging
- water-vapour barriers for protecting phosphor coatings in electroluminescent lamps (the phosphor chemicals are sensitive to moisture)
- protection of liquid-crystal display (LCD) panels, which are sensitive to moisture
Due to its chemical stability, it acts as a protective barrier against chemicals. It is used as a coating and prefabricated liner for chemical applications. PCTFE is also used for laminating other polymers like PVC, polypropylene, PETG, APET etc. It is also used in tubes, valves, chemical tank liners, O-rings, seals and gaskets.[15]
PCTFE is used to protect sensitive electronic components because of its excellent electrical resistance and water repulsion. Other uses include flexible printed circuits and insulation of wires and cables.[23][22]
Low-molecular-weight PCTFE waxes, oils and greases find their application as inert sealants and lubricants. They are also used as gyroscope flotation fluids and plasticizers for thermoplastics.[2]
PCTFE is used for cryogenic seals and components. The cryogenic and liquid gas sector uses mainly PCTFE seals as this material has low gas absorption and good resistance to temperatures below -200 °C.[24]
References
[edit]- ^ a b c d Christopher C. Ibeh (2011). THERMOPLASTIC MATERIALS Properties, Manufacturing Methods, and Applications. CRC Press. p. 491. ISBN 978-1-4200-9383-4.
- ^ a b c d C. H. Kurita (20 Jan 1988). "Appendix A" (PDF). D-ZERO COLD VALUE. pp. 58–61. Archived from the original (PDF) on 21 October 2013. Retrieved June 14, 2012.
- ^ Tsuyoshi Nakajima; Henri Groult (4 August 2005). Fluorinated Materials For Energy Conversion. Elsevier. p. 472. ISBN 978-0-08-044472-7. Retrieved 14 July 2012.
- ^ B. Améduri; Bernard Boutevin (7 July 2004). Well-architectured Fluoropolymers: Synthesis, Properties And Applications. Elsevier. p. 5. ISBN 978-0-08-044388-1. Retrieved 14 July 2012.
- ^ Koch 2012, p. 11.
- ^ a b Takashi Okazoe. "Synthetic Studies on Perfluorinated Compounds by Direct Fluorination" (PDF). p. 17. Retrieved July 14, 2012.
- ^ Suhithi M. Peiris; Gasper J. Piermarini (10 December 2008). Static Compression of Energetic Materials. Springer. pp. 158–. ISBN 978-3-540-68146-5. Retrieved 14 July 2012.
- ^ Sina Ebnesajjad (31 December 2000). Fluoroplastics, Volume 1: Non-Melt Processible Fluoroplastics. William Andrew. p. 74. ISBN 978-0-8155-1727-6. Retrieved 8 July 2012.
- ^ DIANE Publishing Company (1 July 1993). New Materials Society, Challenges and Opportunities: New Materials Science and Technology. DIANE Publishing. p. 8.42. ISBN 978-0-7881-0147-2. Retrieved 8 July 2012.
- ^ Ernst-Christian Koch (17 April 2012). Metal-Fluorocarbon Based Energetic Materials. John Wiley & Sons. p. 23. ISBN 978-3-527-32920-5. Retrieved 8 July 2012.
- ^ ГОСТ 13744-83 State Standard of USSR
- ^ Sina Ebnesajjad (31 December 2002). Melt Processible Fluoropolymers: The Definitive User's Guide and Databook. William Andrew. p. 636. ISBN 978-1-884207-96-9. Retrieved 8 July 2012.
- ^ Ebnesajjad 2000, p. 61.
- ^ a b Ruth Winter (2 August 2007). A Consumer's Dictionary of Household, Yard and Office Chemicals: Complete Information About Harmful and Desirable Chemicals Found in Everyday Home Products, Yard Poisons, and Office Polluters. iUniverse. p. 255. ISBN 978-0-595-44948-4. Retrieved 14 July 2012.
- ^ a b c François Cardarelli (2008). Materials Handbook: A Concise Desktop Reference. Springer. pp. 708–709. ISBN 9781846286681. ISBN 1846286689.
- ^ Ebnesajjad, Sina. Fluoroplastics, Volume 2: Melt Processible Fluoropolymers – The Definitive User Guide and Data Book. p. 560.
- ^ "RIDOUT PLASTICS". Retrieved June 5, 2012.
- ^ J. A. Brydson (8 November 1999). Plastics Materials. Butterworth-Heinemann. pp. 423–. ISBN 978-0-7506-4132-6. Retrieved 30 June 2012.
- ^ Drobny 2006, p. 8, 22.
- ^ "Chapter Two: Sixth Part". Archived from the original on 2012-01-07. Retrieved 2012-06-13.
- ^ "Dielectric Properties of Semicrystalline Polychlorotrifluoroethylene" (PDF). Journal of Research of the National Bureau of Standards Section A. 66A (4): 1. 1962. Retrieved June 26, 2012.
- ^ a b Dominick V. Rosato; Donald V. Rosato; Matthew V. Rosato (2004). Plastic Product Material and Process Selection Handbook. Elsevier. p. 75. ISBN 185617431X. ISBN 9781856174312.
- ^ Drobny 2006, p. 37-39.
- ^ "Technical plastics for cryogenics". Société des Plastiques Nobles. Archived from the original on 2024-07-21. Retrieved 2020-02-14.
